Here you will find information on how to adopt GS1 standards across the three core enablers (people, products and places), how to apply these to specific use cases such as surgical instrument traceability, and details on supporting regulatory requirements.
Take a look at our detailed guidelines for expert advice to support you along the way.
Starting with the core enablers
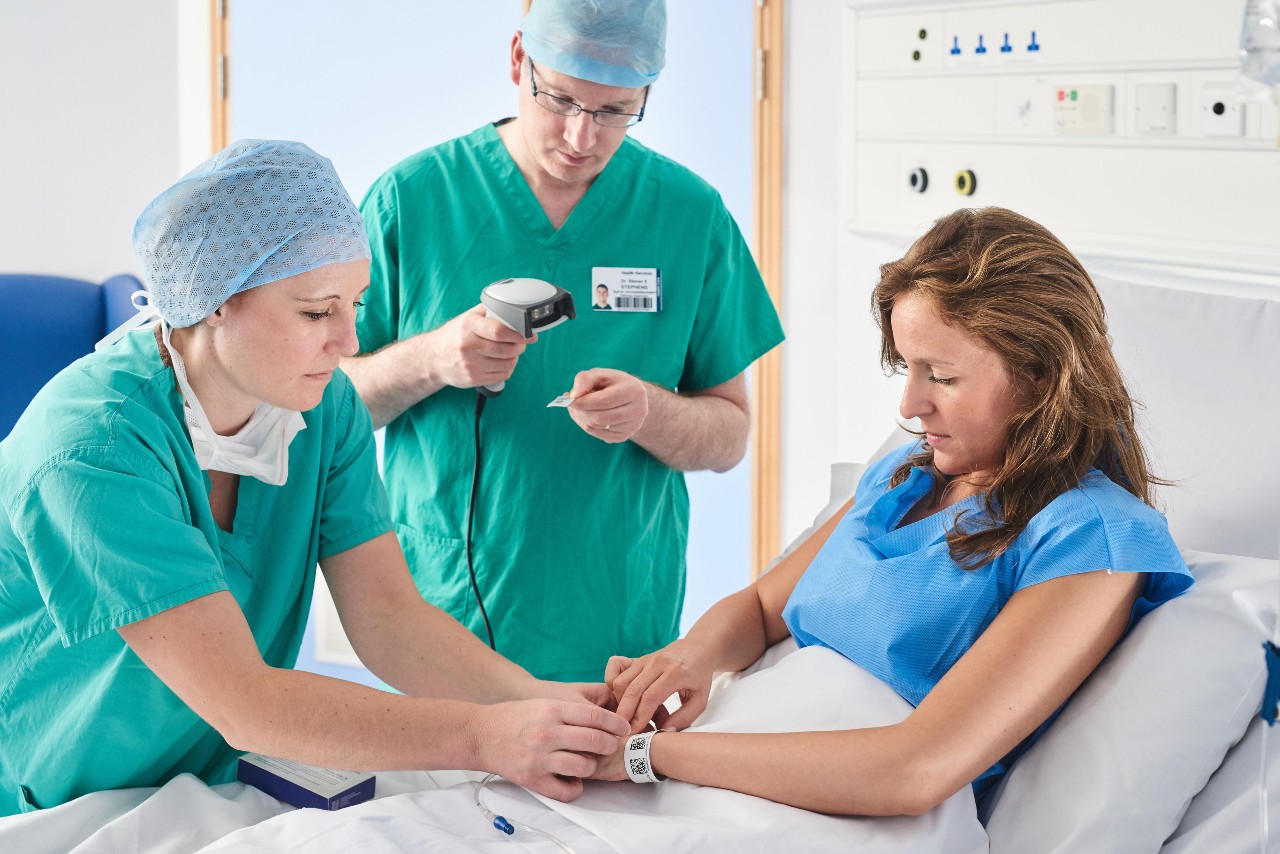
Patient identification
"How-to" guide for using GS1 standards for patient ID.

Staff identification
"How-to" guide for using GS1 standards for staff ID.

Product identification
"How-to" guide for using GS1 standards for product ID.

Place management
"How-to" guide for using GS1 standards for place management.
Getting the use cases right
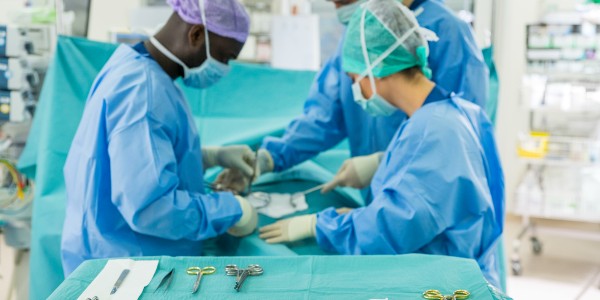
Surgical instrument traceability high-level guide
High-level guide to standards required by systems with surgical-instrument tracking functionality.

Surgical instrument traceability guidance
A guide to using GS1 standards to improve traceability and increase the reliability of data for managing surgical instruments.

Technologies for marking surgical instruments
A guide to the various AIDC technologies that can be used to identify individual surgical instruments.

Inventory management systems guidance
Guidance for healthcare organisations and suppliers for inventory management systems and GS1 standards.
Asset management guidance
A user guide on how to implement asset management processes using GS1 standards.
Standards and implementation

Compliance specification for NHS systems
A guide for hospitals and solution providers on procuring and providing GS1-compliant solutions.

Healthcare GTIN allocation rules
Guidance provided on how to correctly allocate GTINs to products and devices.

GLN allocation rules standard
Best practice guidance for allocating GLNs for location management.
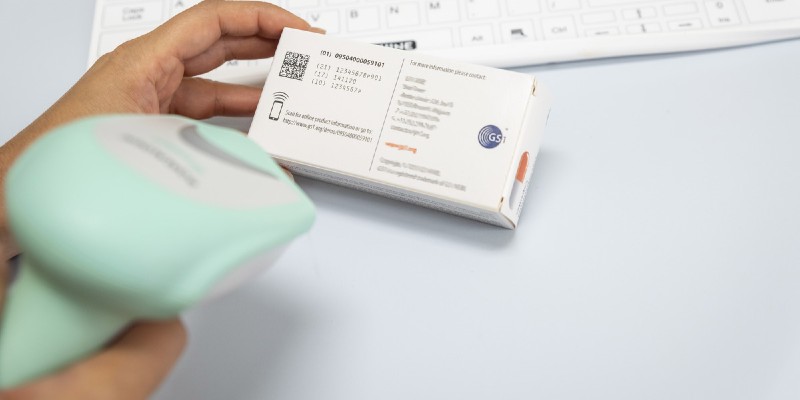
GS1 DataMatrix Guideline
Overview and technical introduction to the use of GS1 DataMatrix.

Supplier adoption "how-to" guide
A best practice guide for healthcare providers when engaging with suppliers.

Barcoding OPCS-4 and ICD-10 Codes GS1 UK Implementation Guidelines
Aligning GS1 standards with coding schemes for procedures and diagnostics.

AIDC healthcare implementation guideline
Comprehensive guidance on how to implement AIDC in healthcare.
DCB1077 for patient wristbands
How to use GS1 standards for AIDC for patient identification.

DAPB0108 for AIDC
Requirements for GS1 standards compliance for the NHS Digital standard DAPB0108.
Regulations

Field Safety Corrective Actions
Recommendations on medical device and IVD FSCAs and recalls using UDI and GS1 standards.
Unique Device Identification (UDI)
How to use GS1 standards to comply with international UDI regulations.

Pharmaceutical regulation for patient safety
Using GS1 standards for pharmaceutical serialisation.
Scan4Safety, case studies and global reference books

The Scan4Safety Report
Full report from the two-year Scan4Safety programme and beyond.

GS1 UK healthcare case study portal
Explore healthcare case studies from GS1 UK members, partners, and suppliers, from across the UK.
GS1 global healthcare reference book 2021-22
Read our 2021-22 healthcare case studies from around the world.
GS1 global healthcare reference book 2020-21
Explore our 2020-21 array of international healthcare case studies.